
Tlr7 drives sex differences in age- and Alzheimer’s disease–related demyelination
Science, 2024
Demyelination, a process observed in aging and age-related neurodegenerative disorders, has shown sex biases in both incidence and severity. Lopez-Lee et al. studied the mechanisms underpinning these sex-based differences in mice using single-nuclei transcriptomics, spatial transcriptomics, and functional analyses. The X-linked gene Tlr7 determined sex differences in demyelination, and its depletion or inhibition reduced sex differences and protected against demyelination in a mouse model of tau-mediated demyelination. These results contribute to elucidating the mechanisms mediating sex differences in neurological disorders. —Mattia Maroso (Editor’s summary)
To read more, click here.
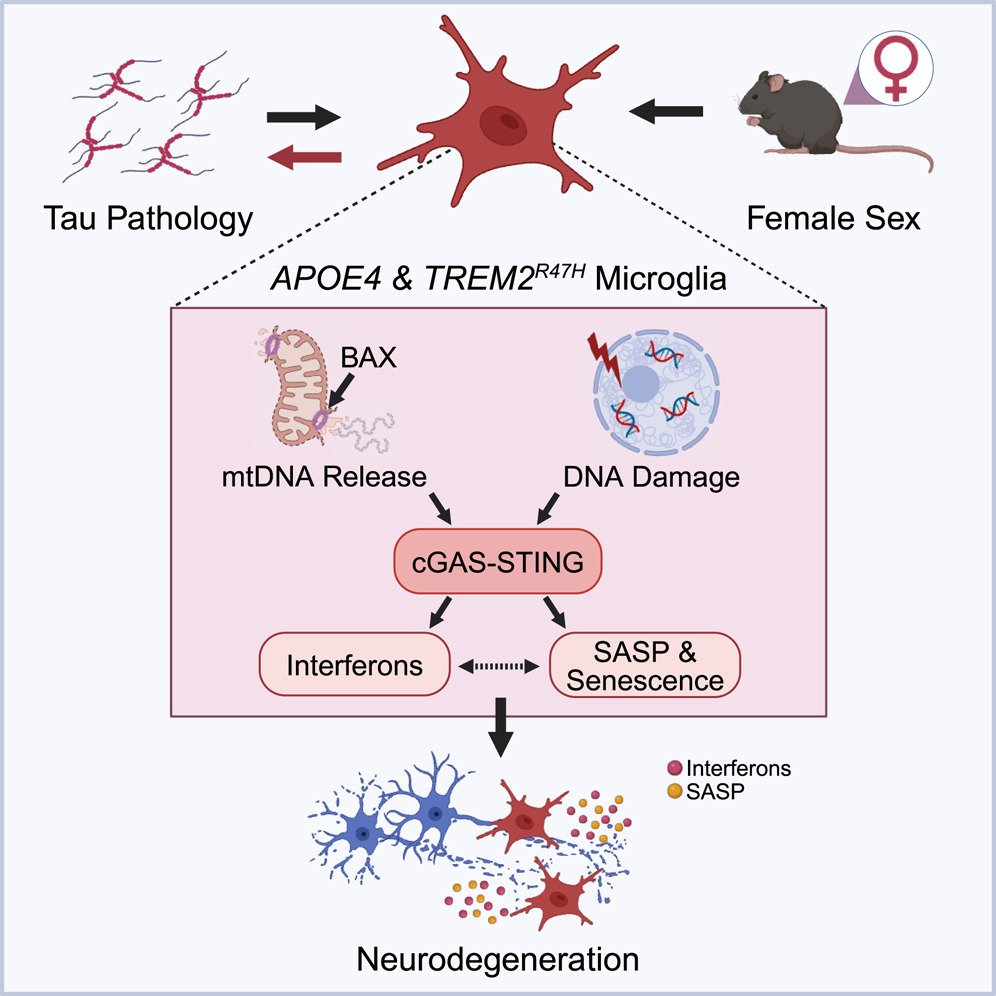
Alzheimer’s disease-linked risk alleles elevate microglial cGAS-associated senescence and neurodegeneration in a tauopathy model
Neuron, 2024
The strongest risk factors for late-onset sporadic Alzheimer’s disease (AD) include the ε4 allele of apolipoprotein E (APOE), the R47H variant of triggering receptor expressed on myeloid cells 2 (TREM2), and female sex. Here, we combine APOE4 and TREM2R47H (R47H) in female P301S tauopathy mice to identify the pathways activated when AD risk is the strongest, thereby highlighting detrimental disease mechanisms. We find that R47H induces neurodegeneration in 9- to 10-month-old female APOE4 tauopathy mice. The combination of APOE4 and R47H (APOE4-R47H) worsened hyperphosphorylated tau pathology in the frontal cortex and amplified tauopathy-induced microglial cyclic guanosine monophosphate (GMP)-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling and downstream interferon response. APOE4-R47H microglia displayed cGAS- and BAX-dependent upregulation of senescence, showing association between neurotoxic signatures and implicating mitochondrial permeabilization in pathogenesis. By uncovering pathways enhanced by the strongest AD risk factors, our study points to cGAS-STING signaling and associated microglial senescence as potential drivers of AD risk. To read more, see here.

Cellular and pathological functions of tau
Nat Rev Mol Cell Biol, 2024
Tau protein is involved in various cellular processes, including having a canonical role in binding and stabilization of microtubules in neurons. Tauopathies are neurodegenerative diseases marked by the abnormal accumulation of tau protein aggregates in neurons, as seen, for example, in conditions such as frontotemporal dementia and Alzheimer disease. Mutations in tau coding regions or that disrupt tau mRNA splicing, tau post-translational modifications and cellular stress factors (such as oxidative stress and inflammation) increase the tendency of tau to aggregate and interfere with its clearance. Pathological tau is strongly implicated in the progression of neurodegenerative diseases, and the propagation of tau aggregates is associated with disease severity. Recent technological advancements, including cryo-electron microscopy and disease models derived from human induced pluripotent stem cells, have increased our understanding of tau-related pathology in neurodegenerative conditions. Substantial progress has been made in deciphering tau aggregate structures and the molecular mechanisms that underlie protein aggregation and toxicity. In this Review, we discuss recent insights into the diverse cellular functions of tau and the pathology of tau inclusions and explore the potential for therapeutic interventions. To read more, see here.

Anti-acetylated-tau immunotherapy is neuroprotective in tauopathy and brain injury
Molecular Neurodegeneration, 2024
In the latest study from the Gan Lab, published in Molecular Neurodegeneration, Parra Bravo, Krukowski, and Barker et al. report two newly-generated anti-acetylated-tau-K174 antibodies that effectively mitigate neurobehavioral impairments and reduce pathology in PS19 mice— alone and in conjunction with traumatic brain injury (TBI).
The collaborative study was led by Celeste Parra Bravo from the Gan laboratory, Dr. Karen Krukowski from Dr. Susanna Rosi’s group (UCSF), and Sarah Barker from Dr. Andrew Pieper’s group (University Hospitals Cleveland Medical Center), with Dr. Rosi, Dr. Xu Chen (UCSD), and Dr. Li Gan as senior investigators.
Immunotherapy of mice harboring the P301S tau mutation (PS19) with anti-ac-tauK174 antibody rescued neurobehavioral impairments, and ameliorated neuropathology and neurodegeneration. Ac-tauK174 increased significantly in human plasma 24 hr after TBI, and anti-ac-tauK174 treatment of PS19 mice blocked TBI-induced neurodegeneration, preserved memory functions, and rescued alterations of microglial and oligodendrocyte transcriptomic states.
Read the full paper HERE.
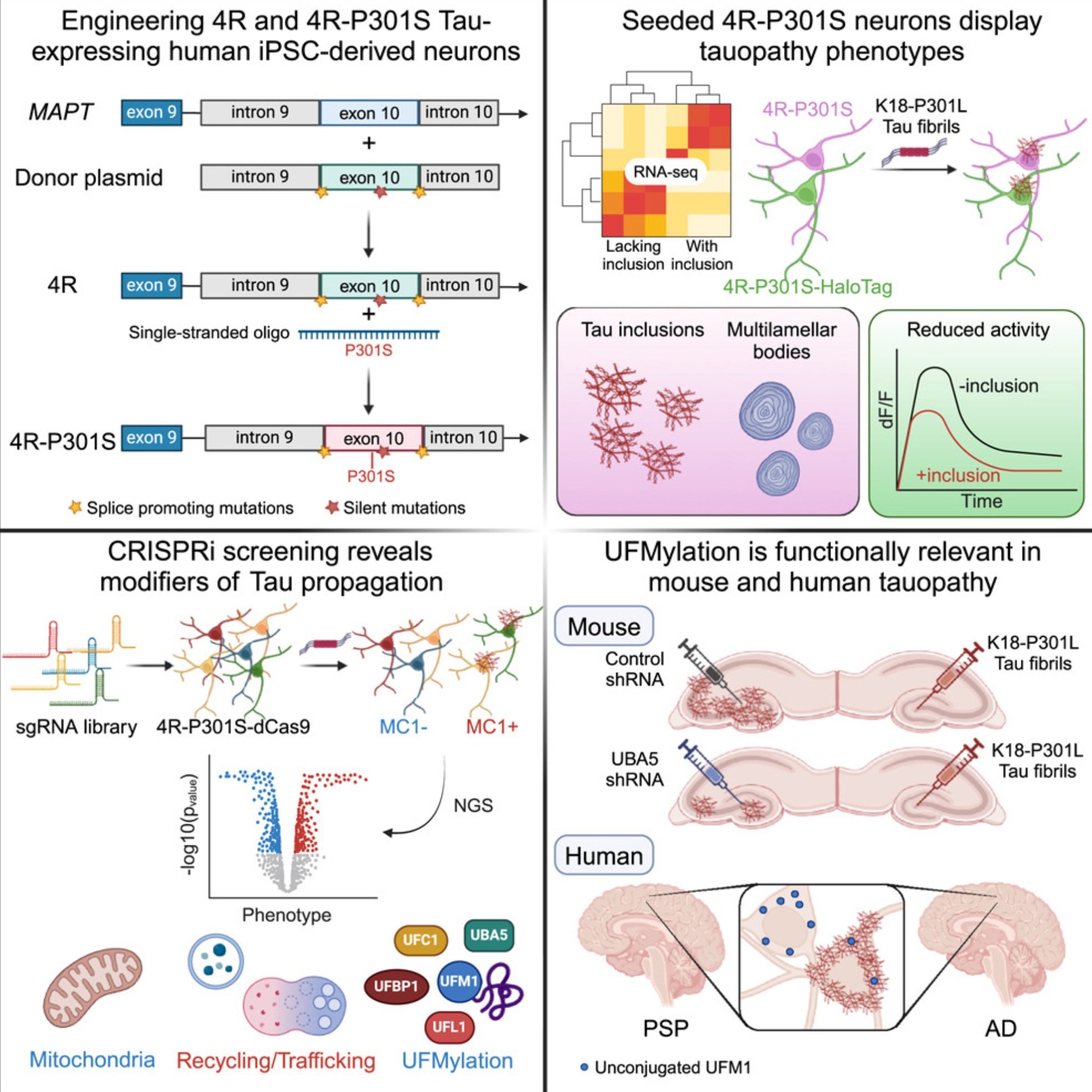
Human iPSC 4R tauopathy model uncovers modifiers of tau propagation
Cell, 2024
Tauopathies are age-associated neurodegenerative diseases whose mechanistic underpinnings remain elusive, partially due to a lack of appropriate human models. Here, we engineered human induced pluripotent stem cell (hiPSC)-derived neuronal lines to express 4R Tau and 4R Tau carrying the P301S MAPT mutation when differentiated into neurons. 4R-P301S neurons display progressive Tau inclusions upon seeding with Tau fibrils and recapitulate features of tauopathy phenotypes including shared transcriptomic signatures, autophagic body accumulation, and reduced neuronal activity. A CRISPRi screen of genes associated with Tau pathobiology identified over 500 genetic modifiers of seeding-induced Tau propagation, including retromer VPS29 and genes in the UFMylation cascade. In progressive supranuclear palsy (PSP) and Alzheimer’s Disease (AD) brains, the UFMylation cascade is altered in neurofibrillary-tangle-bearing neurons. Inhibiting the UFMylation cascade in vitro and in vivo suppressed seeding-induced Tau propagation. This model provides a robust platform to identify novel therapeutic strategies for 4R tauopathy.

Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy
Nature Communications, 2022
Activation of microglia is a prominent pathological feature in tauopathies, including Alzheimer’s disease. How microglia activation contributes to tau toxicity remains largely unknown. Here we show that nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, activated by tau, drives microglial-mediated tau propagation and toxicity. Constitutive activation of microglial NF-κB exacerbated, while inactivation diminished, tau seeding and spreading in young PS19 mice. Inhibition of NF-κB activation enhanced the retention while reduced the release of internalized pathogenic tau fibrils from primary microglia and rescued microglial autophagy deficits. Inhibition of microglial NF-κB in aged PS19 mice rescued tau-mediated learning and memory deficits, restored overall transcriptomic changes while increasing neuronal tau inclusions. Single cell RNA-seq revealed that tau-associated disease states in microglia were diminished by NF-κB inactivation and further transformed by constitutive NF-κB activation. Our study establishes a role for microglial NF-κB signaling in mediating tau spreading and toxicity in tauopathy.

A MAC2-positive progenitor-like microglial population is resistant to CSF1R inhibition in adult mouse brain
eLife, 2020
Microglia are the resident myeloid cells in the central nervous system (CNS). The majority of microglia rely on CSF1R signaling for survival. However, a small subset of microglia in mouse brains can survive without CSF1R signaling and reestablish the microglial homeostatic population after CSF1R signaling returns. Using single-cell transcriptomic analysis, we characterized the heterogeneous microglial populations under CSF1R inhibition, including microglia with reduced homeostatic markers and elevated markers of inflammatory chemokines and proliferation. Importantly, MAC2/Lgals3 was upregulated under CSF1R inhibition, and shared striking similarities with microglial progenitors in the yolk sac and immature microglia in early embryos. Lineage-tracing studies revealed that these MAC2+ cells were of microglial origin. MAC2+ microglia were also present in non-treated adult mouse brains and exhibited immature transcriptomic signatures indistinguishable from those that survived CSF1R inhibition, supporting the notion that MAC2+ progenitor-like cells are present among adult microglia.